In the process of Brownian motion, inorganic nanoparticles self-organize into a variety of complex architectures. In fact, this ability is common for all nanostructures regardless whether they are made from organic or inorganic matter. Its generality originates from the correlated symmetry-dependent movements of nanoscale particles subject to thermal motion. Furthermore, effective long-range attraction is stronger for more polarizable (metallic and semiconducting) than for less polarizable (organic, insulating) particles. Hence self-assembly phenomena for inorganic nanoparticles typically occur under a wider range of conditions than for organic ones. The amplified effects of subtle nanoscale anisotropies (such as chirality), collective motion, and non-additivity of their interactions further facilitate their self-organization that can lead to amazingly complex biomimetic structures.
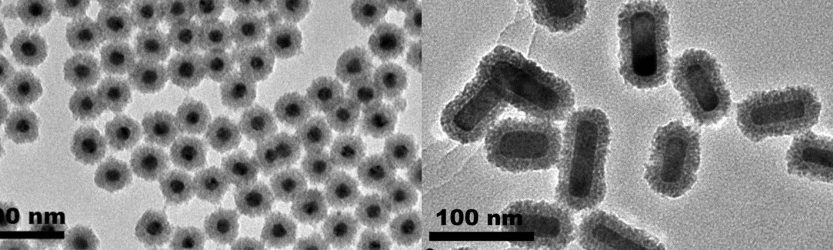
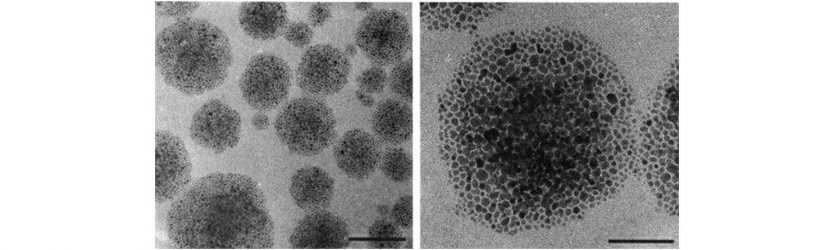
Simple water-soluble nanoparticles can form an immense variety of assemblies. Some of them are limited to specific size in one growth direction, some are unlimited and can reach macroscale dimensions. The first class of assemblies is exemplified by supraparticles, nanoshells, nanohelices, etc. They are formed by 50-1000 individual particles and, in many ways, similar to micelles, vesicles, and viruses. The hierarchical organization of many of these assemblies rival in complexity to size-limited assemblies found in biology, exemplified by virus-like nanoshells, collagen-like nanohelices, and coccolith-like spiky supraparticles.
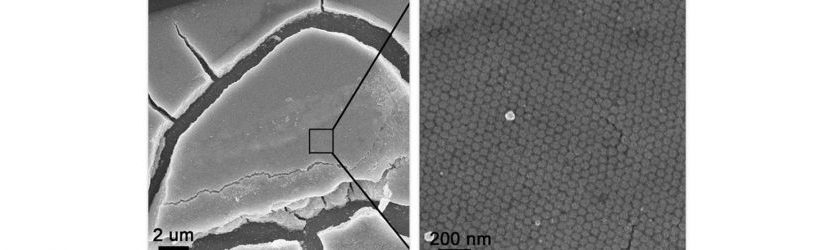
Slight variations of nanoparticle chemistry and assembly conditions lead to switching of one self-organization pattern to another. The same particles forming, for instance nanohelices, can be guided to produce the second class of nanoassemblies that display at least one unrestricted dimension. They are exemplified by chains, nanowires, sheets, ribbons, multilayers, and superlattices. This class of assemblies made from nanoscale metals and semiconductors gives rise to a wide variety of composites with unique combination of properties. They are used in a variety of energy storage materials, biosensing protocols, and optoelectronic devices.
In our ongoing studies, we are addressing the following questions:
What are the limits of structural complexity for nanoparticle superstructures?
How to enumerate the intermolecular forces essential for nanoparticle interactions?
How to extend the current physicochemical toolbox for nanoparticle system in thermodynamic equilibrium to those in dynamic states in open systems?
Answering these questions about nanoparticle assemblies sheds light on inter-particle forces in additive (DLVO-abiding) and non-additive (DLVO-violating) regimes. The utilization of ‘free’ thermal energy to create complex functional systems vividly highlights the practicality of self-assembly processes and their importance to current and future energy efficiency, for instance, in self-assembly of hierarchical biomimetic composites.